The Bee Community of Wooster’s Pollinator Plots
Webpage produced by: Dan Borowsky, Emily Greenland, and Charles Burrows
Bee-Search Introduction

Students at the College of Wooster, mentored by Professor Jennifer Ison, undertook research to learn about the bee population around areas of native plantings called pollinator gardens. Students placed bowl traps around the gardens and collected bees from the spring through early fall. Students then pinned and identified the bees, after which they documented each bee on iNaturalist and analyzed trends in the bee populations. This work helps researchers understand the health of bee communities.
Methods
– Collecting Specimens

Researchers at Wooster selected bee bowls, also called pan or Moericke traps, to capture bees. Bee bowls are a brightly colored white, blue, or yellow bowl that is filled with soapy water. Bees are attracted to the bowl because they perceive it to be a flower. Both have a round shape, and share similar colors, and are UV reflective. The soapy water causes them to drown when they fly in by removing surface tension.(1) Other options for catching bees, such as catching bees in a net, or direct observation, were rejected as being too labor intensive and too inaccurate respectively.
 Researchers used bee bowls due to their ease of use and repeatability. Bee bowls require no human interaction other than collecting bees from the traps and refilling the water, and they thus provide none of the uncertainty that netting or observation creates, as those methods rely on the skill of individual researchers. They placed bowls in 15 sites around the College of Wooster Campus. Locations were chosen to maximize the area covered, and bowls were placed in triads with one each of a white, yellow, and blue bowl.
Researchers used bee bowls due to their ease of use and repeatability. Bee bowls require no human interaction other than collecting bees from the traps and refilling the water, and they thus provide none of the uncertainty that netting or observation creates, as those methods rely on the skill of individual researchers. They placed bowls in 15 sites around the College of Wooster Campus. Locations were chosen to maximize the area covered, and bowls were placed in triads with one each of a white, yellow, and blue bowl.

Research suggests that bee bowls disproportionately collect larger numbers of bees from Halictidae than from other families, and that about 30 bowls is necessary to determine species richness in a habitat.(1,2) This is reinforced by a study that demonstrates that bowls and nets catch different sections of the bee population.(3) This data cannot therefore be used to indicate species richness, or overall population size. The data collected at Wooster is therefore relevant in determining the change in population size of commonly captured bee species in the study area over the time the bees were collected.
– Analyzing Data
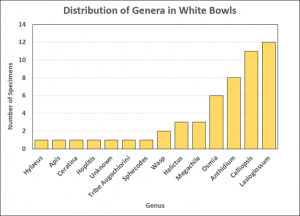 Once bees were recorded, pinned, and identified, their information was put into a spreadsheet. This allowed researchers to easily sort through data and create visualizations such as this graph (right) to look at possible trends in visitation rates. There have been numerous studies surrounding pan traps, some with conflicting results. Common studies examine trends in visitation rates and attempt to determine factors that may influence these rates, such as bowl color or surrounding flower color (4,5). While one season’s worth of data from Wooster’s plots may not be enough to truly study these trends, it does hint at possible future directions for research in and around our pollinator gardens.
Once bees were recorded, pinned, and identified, their information was put into a spreadsheet. This allowed researchers to easily sort through data and create visualizations such as this graph (right) to look at possible trends in visitation rates. There have been numerous studies surrounding pan traps, some with conflicting results. Common studies examine trends in visitation rates and attempt to determine factors that may influence these rates, such as bowl color or surrounding flower color (4,5). While one season’s worth of data from Wooster’s plots may not be enough to truly study these trends, it does hint at possible future directions for research in and around our pollinator gardens.

 After analyzing the data from late May to late July, researchers found that Genus Anthidium was only found in white bee bowls (8 in white, 0 in blue or yellow out of 129 bees). Bees in the Anthidium genus harvest nesting material from plants with an abundance of hair-like fibers (like those in Lamiaceae, the mint family) and get nectar from a wide variety of plants (6). Perhaps Anthidium in the area were searching for plants with whitish fibers or flowers- like the white mountain mint in our garden- and got trapped in white bowls instead? This is just one of many possible questions stemming from our data that could inspire further research. (Visit the “Types of Bees” section below for more Anthidium information).
After analyzing the data from late May to late July, researchers found that Genus Anthidium was only found in white bee bowls (8 in white, 0 in blue or yellow out of 129 bees). Bees in the Anthidium genus harvest nesting material from plants with an abundance of hair-like fibers (like those in Lamiaceae, the mint family) and get nectar from a wide variety of plants (6). Perhaps Anthidium in the area were searching for plants with whitish fibers or flowers- like the white mountain mint in our garden- and got trapped in white bowls instead? This is just one of many possible questions stemming from our data that could inspire further research. (Visit the “Types of Bees” section below for more Anthidium information).

 Researchers also found that Osmia species were mostly only present in the sampling in late May. These bees are great fruit tree pollinators and are even kept by some farmers and gardeners for their pollination help (7). There were 7 Osmia collected at Wooster on 5/26, none on 6/9, and one Osmia on 6/23, after which no more were collected for the year. These findings are consistent with the fact that the season for Osmia is early springtime (7). This is an example of how local bee data can help study the seasonality and behavior of bees in the area, especially when paired with other studies or data (like the community data on iNaturalist).
Researchers also found that Osmia species were mostly only present in the sampling in late May. These bees are great fruit tree pollinators and are even kept by some farmers and gardeners for their pollination help (7). There were 7 Osmia collected at Wooster on 5/26, none on 6/9, and one Osmia on 6/23, after which no more were collected for the year. These findings are consistent with the fact that the season for Osmia is early springtime (7). This is an example of how local bee data can help study the seasonality and behavior of bees in the area, especially when paired with other studies or data (like the community data on iNaturalist).
Types of Bees Found at the plots
– Family Halictidae

Metallic Sweat Bee (Subgenus Dialictus)
This is the second largest of all the bee families, and bees that make up this family are known as sweat bees. There are over 2000 species of sweat bees and describing them can be difficult due to their vast abundance. Notably most species in this family are brown or black with yellow stripes, however others are metallic in appearance and can range from green to blue to red in color. These bees are mainly polylectic, or gathering from a variety of flowers, with a small percentile being parasitic towards other bees and instead relying on their nests for food. All sweat bees can be differentiated from other bees due to having an arcuate basal vein which runs diagonally through the radial wing cells of these bees. Their abundance gives them a variety of ecosystem functions, however their small size makes them quite capable of nectar robbing and generally poor pollinators.
– Genus Megachile

Two-spotted Longhorn Bee (Melissodes bimaculatus)
This genus of bees is typically called the leaf cutter bees, as they are sometimes seen snipping off small pieces of plants and flying them back to their nests. These solitary bees are known to inhabit small cavities especially those within dead trees. Leaf cutter bees are mostly generalist pollinators which gather resources from a variety of plant species. Defining characteristics of these bees are the abundance of bumps and other characteristics on their bodies, as well as much larger mandibles to help them bite through plant tissues.
– Genus Anthidium

Oblong Woolcarder Bee (Anthidium oblongatum)
This genus, also commonly called the Wool Carder Bees, is uniquely more brightly colored than other bees giving them a fly or wasp-like appearance. Similarly these bees also tend to hover around flowers and have been seen defending specific inflorescences. Female bees in the genus Anthidium line their nests (typically burrows underground or within structures) with a thick fluffy material which is produced from the bees chewing off the small hairs which grow from some plant stems. This unique trait provides these bees with unique wool-covered nests which stand out from the nests made by other solitary bees.

Wool Carder Bee – nest building – Anthidium manicatum – Female
– Genus Nomada

Unknown Nomad Bee Specimen
This genus is known as the nomad bees, as they are solitary bees which do not produce nests of their own. Nomad bees are kleptoparasitic meaning they rely on the nests of other bees to lay their eggs and also sometimes steal the food stored within as well. For this reason, they are also known as cuckoo bees. Their behavioral inclination to find previously-gathered food makes them generally poor pollinators, however they do sometimes land on flowers for nectar. These bees are smaller on average and appear wasp-like, lacking hair and often with bright colors.
Image Citations:
Bee Images Provided By Emily Greenland’s INaturalist
Pollinator Plot images provided by Jennifer Ison
Ilona L. (July 4, 2010). Wool Carder Bee – nest building – Anthidium manicatum – Female BugGuide.net https://bugguide.net/node/view/421613
Sweat Bee (family Halictidae).Caddo Lake, Harrison Co., Texas.N62.68279 W94.1508.May 31, 2011..Public Domain image by Christopher Johnson. Part of the “Insects Unlocked” Project. University of Texas at Austin
References:
- Alexander, B. A. 1994. Species-groups and cladistic analysis of the cleptoparasitic bee genus Nomada (Hymenoptera: Apoidea). University of Kansas Science Bulletin 55: 175-238.
- Buckley, K., Nalen, C. Z., & Ellis, J. (2011). Sweat Bees, halictid bees, Halictidae (Insecta: Hymenoptera: Halictidae). EDIS, 2011(8). https://doi.org/10.32473/edis-in897-2011
- Gallagher, Samantha and Lucky, Andrea. 2019. European Wool Carder Bee, Anthidium manicatum (Linnaeus) (Insecta: Hymenoptera: Megachilidae). Entomology and Nematology Department, UF/IFAS Extension.
- Grundel, Ralph, et al. “Effectiveness of Bowl Trapping and Netting for Inventory of a Bee Community.” Environmental Entomology, vol. 40, no. 2, 2011, pp. 374-380. OhioLINK Electronic Journal Center, doi:10.1603/EN09278
- Hall, H. Glenn. 2016. Color Preferences of Bees Captured in Pan Traps. Journal of the Kansas Entomological Society 89(3):273–76.
- Moisset, Beatriz and Wojcik, Vicki. Blue Orchard Mason Bee (Osmia lignaria). U.S. Forest Service Pollinator of the Month.
- Shapiro, L.H., Tepedino, V.J. & Minckley, R.L. Bowling for bees: optimal sample number for “bee bowl” sampling transects. J Insect Conserv 18, 1105–1113 (2014). https://doi.org/10.1007/s10841-014-9720-y
- Toler, Trent R., Evans, Edward W., Tepedino, Vincent J. 2005. Pan-trapping for bees (Hymenoptera: Apiformes) in Utah’s West Desert: the importance of color diversity. The Pan-Pacific Entomologist 81(3/4):103-113.
- Wilson, J., & Carril, M. (2016). The bees in your backyard: A guide to North America’s bees. Princeton University Press.
- Zachary M Portman, Bethanne Bruninga-Socolar, Daniel P Cariveau, The State of Bee Monitoring in the United States: A Call to Refocus Away From Bowl Traps and Towards More Effective Methods, Annals of the Entomological Society of America, Volume 113, Issue 5, September 2020, Pages 337–342, https://doi.org/10.1093/aesa/saaa010
Testimony of Emily Greenland, Jennifer Ison